Präzise Temperaturmessungen sind in vielen Industriebereichen von entscheidender Bedeutung, insbesondere in der Pharmaindustrie, Kryotechnik und wissenschaftlichen Forschung.
Die Kalibrierung von Thermometern bei extrem niedrigen Temperaturen, die von -80 °C bis -180 °C reichen, stellt eine erhebliche technische Herausforderung dar. Dieser Artikel beschreibt die technischen Möglichkeiten, um Thermometer bei diesen extrem niedrigen Temperaturen zu kalibrieren.
Inhalt
Kalibrierverfahren bei niedrigen Temperaturen
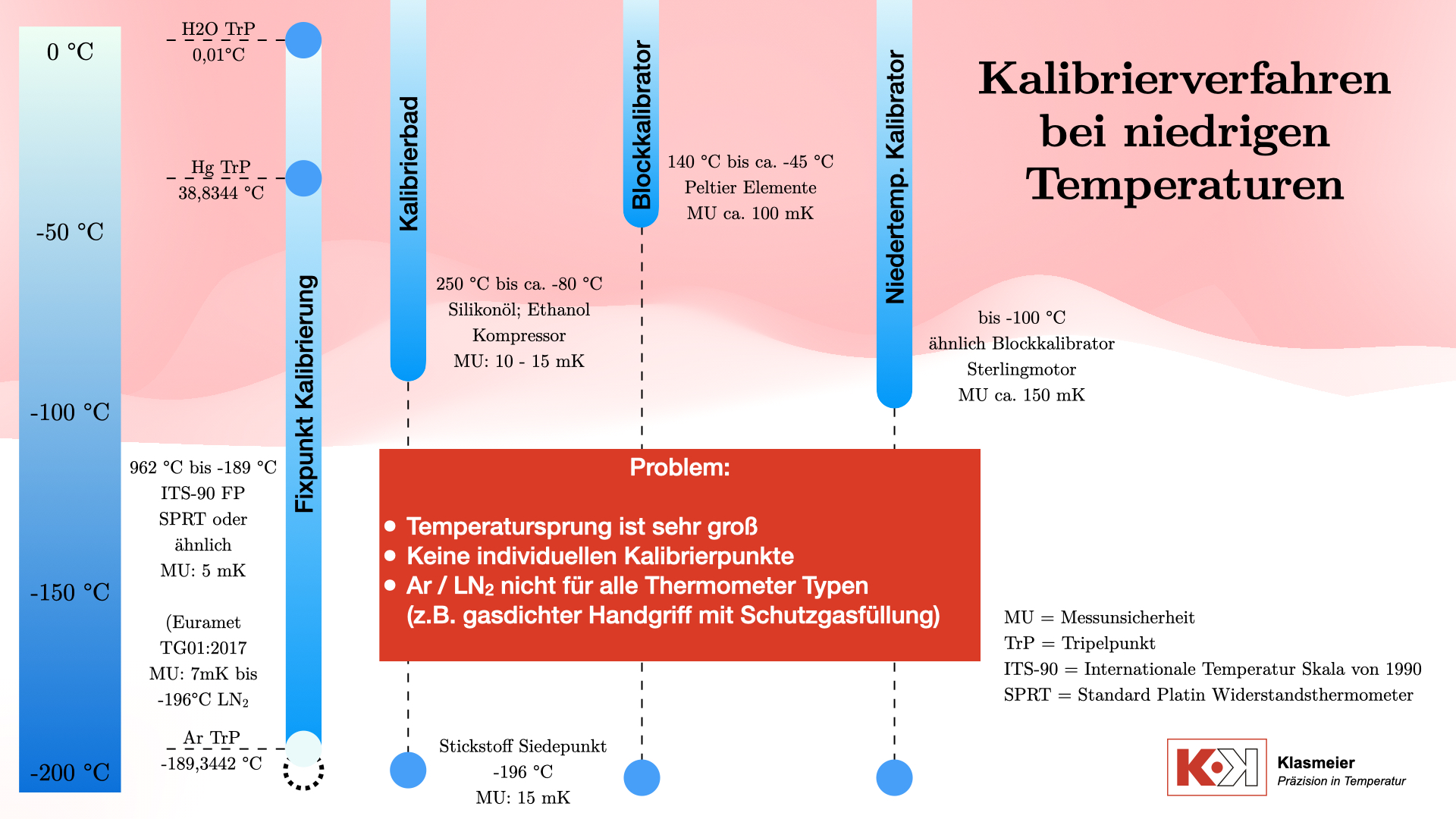
Kalibrierbäder
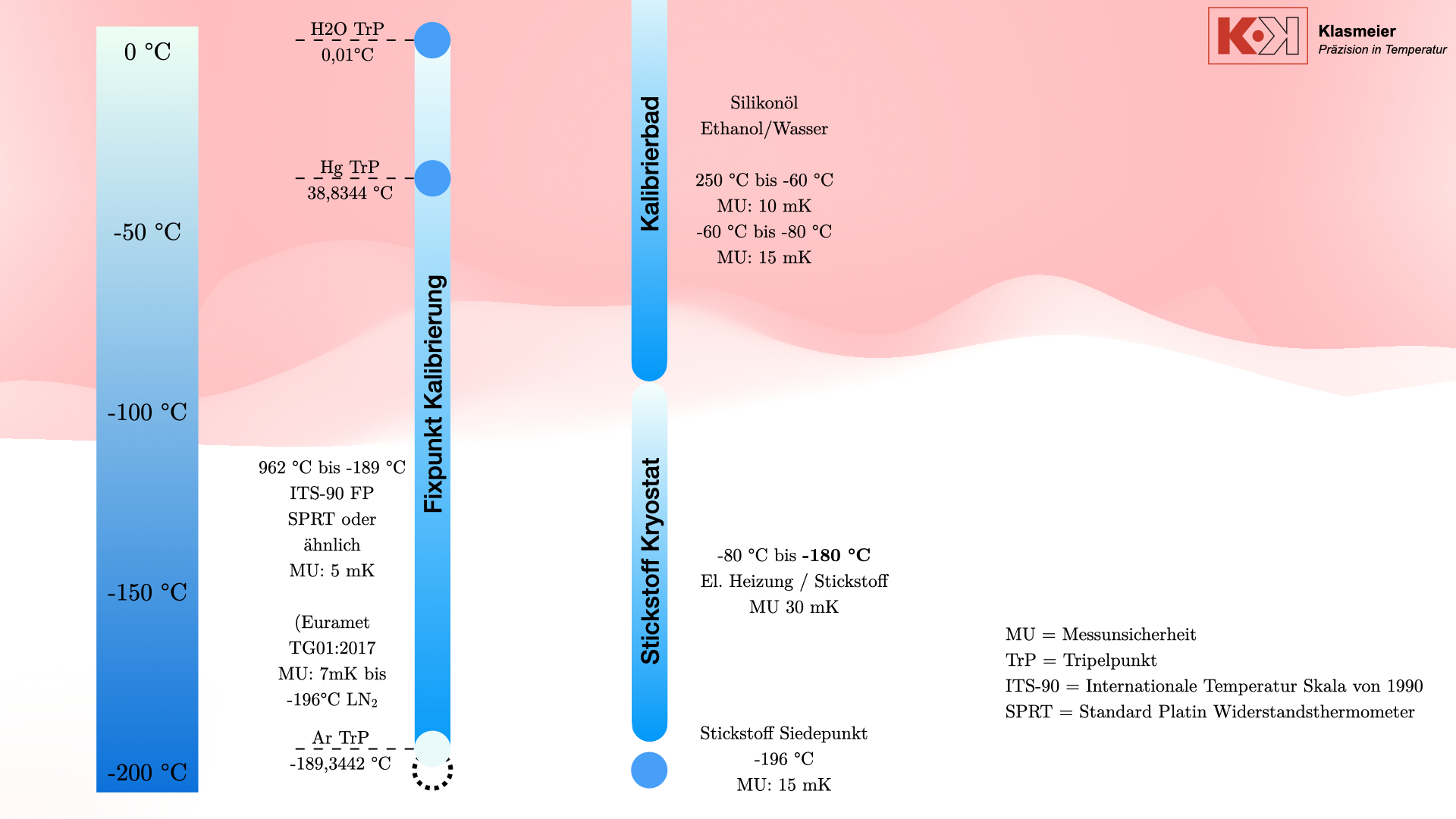
Das klassische Verfahren zur Kalibrierung von Thermometern bei niedrigen Temperaturen verwendet Kalibrierbäder. Diese bestehen typischerweise aus zwei Arbeitskammern:
- Kalibriermediumkammer: In dieser Kammer wird das Kalibriermedium temperiert und zirkuliert.
- Kalibrierkammer: In dieser Kammer wird das Thermometer kalibriert.
Das gebräuchlichste Kalibriermedium ist Silikonöl, das für Temperaturen von bis zu 250 °C verwendet wird. Für niedrigere Temperaturen, bis etwa -80 °C, wird Ethanol verwendet. Einige Kalibrierbäder können sogar noch niedrigere Temperaturen erreichen, doch der Aufwand steht oft nicht im Verhältnis zum Nutzen. Kalibrierbäder werden mittels eines Kompressors gekühlt. Dieser Kompressor läuft immer auf 100 % Leistung, und eine elektrische Heizung temperiert gegen diese volle Kühlleistung. Die Kalibriertemperatur wird also nicht durch das Kühlen erreicht, sondern durch das Heizen gegen die Kühlung. Das hat den Vorteil, dass eine deutlich höhere Regelgenauigkeit beziehungsweise Stabilität erreicht werden kann. Der Nachteil dieser Technik ist der große Aufwand, der betrieben werden muss, um eine stabile Temperatur zu erreichen.
Die erreichbare Messunsicherhei dieser Kalibrierbäder liegt typischerweise bei 10 mK bis 15 mK.
Temperatur Blockkalibratoren
Temperatur-Blockkalibratoren sind in der Industrie aufgrund ihrer Benutzerfreundlichkeit weit verbreitet. Sie arbeiten mit Peltier-Elementen und können Temperaturen bis etwa -70 °C unter der Umgebungstemperatur erreichen. Dadurch ist der Temperaturbereich im negativen Bereich auf etwa -45 °C begrenzt, wenn sie in Laborumgebungen verwendet werden. Ihre Messunsicherheit liegt bei etwa 50 mK bis 100 mK, was für viele industrielle Anwendungen ausreichend ist. Für den Einsatz im Kalibrierlabor ist diese Messunsicherheit nicht genau genug ist.
Niedertemperaturkalibratoren
Niedertemperaturkalibratoren ähneln im Aufbau üblichen Temperatur-Blockkalibratoren. Sie arbeiten jedoch nicht mit Peltier-Elementen, sondern verwenden Stirling-Motoren zur Kühlung des Kalibriervolumens. Sie können Temperaturen bis zu -100 °C erreichen. Diese Geräte haben den Vorteil, dass keine Flüssigkeiten als Kalibriermedium verwendet werden. Sie sind jedoch verhältnismäßig hochpreisig und haben eine hohe Messunsicherheit von etwa 150 mK.
Kalibrierung am sekundären Temperatur-Fixpunkt Stickstoff
Neben den klassischen Kalibrierverfahren gibt es die Möglichkeit, Thermometer am sekundären Temperatur-Fixpunkt Stickstoff zu kalibrieren. Der Siedepunkt von Stickstoff liegt bei -196 °C und stellt einen gut definierten sekundären Fixpunkt dar, der technisch zur Kalibrierung genutzt werden kann. Dieses Verfahren hat jedoch auch einige Nachteile.
Obwohl die Kalibrierung am Stickstoff-Siedepunkt technisch durchführbar und sehr kostengünstig realisierbar ist, ist dieser Punkt nicht in der Internationalen Temperaturskala von 1990 (ITS-90) definiert. Das bedeutet, dass die Kalibrierung nicht die gleiche Genauigkeit und internationale Anerkennung erreicht wie die Temperatur-Fixpunktkalibrierung nach ITS-90.
Ein weiteres Problem ist die begrenzte Anzahl an Kalibrierpunkten. Bei Verwendung des Stickstoff-Siedepunkts stehen zu wenige Kalibrierpunkte zur Verfügung. Der Temperaturabstand von den ca. -80 °C oder -100 °C in bspw. einem Kalibrierbad bis zum Stickstoff-Siedepunkt bis -196 °C ist zu groß um eine präzise Thermometer-Kennlinie berechnen zu können. Eine genaue und kontinuierliche Kalibrierung über den gesamten Temperaturbereich ist daher nicht möglich.
Die Kalibrierung am Stickstoff-Siedepunkt ist daher nicht zu empfehlen, obwohl sie technisch machbar ist.
Temperatur Fixpunktkalibrierung nach der ITS-90
Die präziseste Methode zur Kalibrierung von Thermometern ist die Fixpunktkalibrierung nach der Internationalen Temperaturskala von 1990 (ITS-90). Diese Methode umfasst die folgenden Temperaturfixpunkte Fixpunkte im negativen Temperaturbereich:
- Wasser-Tripelpunkt: 0,01 °C
- Quecksilber-Tripelpunkt: -38,8344 °C
- Argon-Tripelpunkt: -189,3442 °C
Zusätzlich kann der Stickstoff-Siedepunkt bei -196 °C als sekundärer Temperatur-Fixpunkt verwendet werden. Mit der Richtlinie EURAMET TG 01:2017 kann dann die ITS-90 Thermometer-Kennlinie (Abweichungsfunktion) bis -196 °C extrapoliert werden.
Ein Hauptproblem bei der Kalibrierung bei extrem niedrigen Temperaturen an Temperatur-Fixpunkten ist die Handhabung der Thermometer. Spezielle gasdichte Handgriffe und Schutzgasfüllungen sind notwendig, um präzise Messungen zu gewährleisten.
Diese Methode deckt den gesamten Temperaturbereich ab, hat jedoch den Nachteil, dass nicht alle Thermometer für die Fixpunktkalibrierung geeignet sind. Dies führt zu einer Lücke in der Kalibrierfähigkeit für bestimmte Temperaturbereiche und Thermometertypen. Die Thermometer müssen von der Bauform her an Temperatur-Fixpunkten kalibrierfähig sein, d.h. sie müssen eine Mindestlänge aufweisen und einen passenden Durchmesser am Schutzrohr haben.
Außerdem besteht die große Einschränkung, dass die Thermometer der extrem niedrigen Temperatur von ca. -189 °C am Tripelpunkt von Argon ausgesetzt werden müssen. Damit das möglich ist, sind einige bauartbedingte Voraussetzungen am Thermometer notwendig. Zum Beispiel darf sich im Messkanal des Thermometers keine Luft befinden, da der Phasenübergang von Sauerstoff eine Kalibrierung unmöglich machen würde. Diese Tatsache schränkt viele Thermometer ein. Ein Thermometer, das z.B. nur bis -150 °C verwendet werden darf, ist somit an Temperatur-Fixpunkten im negativen Bereich nicht kalibrierfähig.

In eigener Sache
Präzise Kalibrierung bei extrem niedrigen Temperaturen
Mit den Kalibrierdienstleistungen von Klasmeier können Sie Ihre Thermometer im Temperaturbereich von -180 °C bis -80 °C sowie bei den fixen Temperaturen von -189 °C und -196 °C nach DIN EN ISO/IEC 17025 (DAkkS) kalibrieren lassen. Klasmeier bietet Ihnen präzise und zuverlässige Messergebnisse, die Ihren Anforderungen entsprechen.
Herausforderung: Der Phasenübergang von Luft
Ein zentrales Problem bei der Kalibrierung bei extrem niedrigen Temperaturen ist der Phasenübergang der Luft. Bei Temperaturen unter -180 °C beginnen die Bestandteile der Luft (hauptsächlich Stickstoff und Sauerstoff) zu kondensieren und schließlich zu verflüssigen. Dies führt zu erheblichen Messproblemen, da sich das Gasgemisch in verschiedenen Phasen befindet.
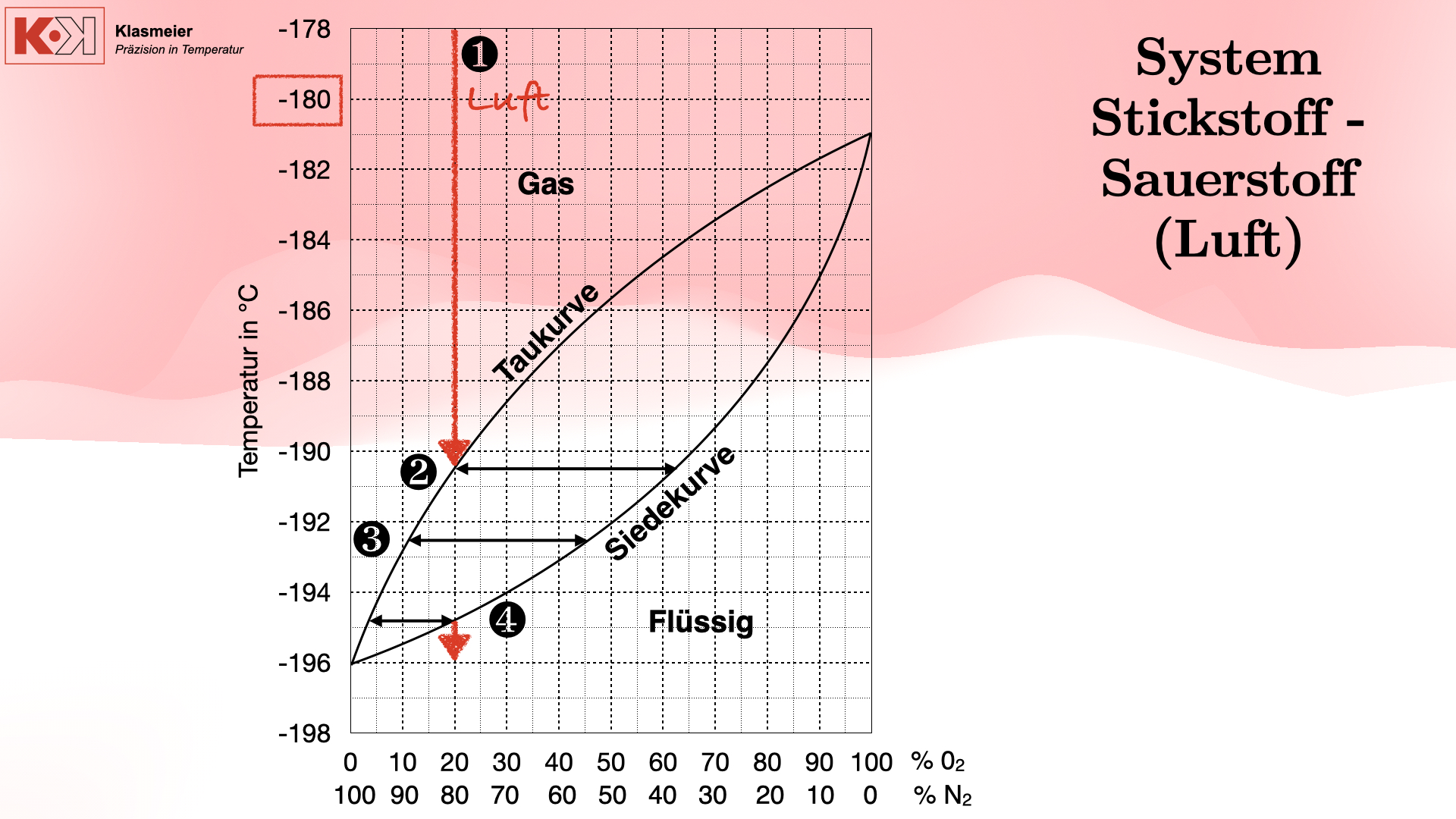
Phasenübergang von Luft:
- Taupunkt: Bei Abkühlung der Luft bilden sich zunächst Tröpfchen aus flüssigem Sauerstoff und Stickstoff, wobei der Taupunkt nicht einheitlich ist, da es sich um ein Gasgemisch handelt.
- Siedepunkt: Weiteres Abkühlen führt zur vollständigen Verflüssigung der Luftbestandteile, jedoch mit unterschiedlichen Zusammensetzungen.
- Kondensation: Schließlich kondensiert die Luft vollständig zu einem flüssigen Gemisch aus 80 % Stickstoff und 20 % Sauerstoff.
Dieser Phasenübergang kann dazu führen, dass Thermometer, die durch diesen Bereich geführt werden, unzuverlässige Messungen liefern, da sie verschiedenen Phasenübergängen unterliegen.
Lösung: Schutzgas und gasdichte Handgriffe
Der Phasenübergang von Luft muss im Thermometer verhindert werden, da ein luftgefülltes Thermometer bei extrem niedrigen Temperaturen nicht funktioniert. Eine Möglichkeit, dieses Problem zu lösen, besteht darin, die Thermometer mit Schutzgas zu befüllen:
- Schutzgasfüllung: Wir verwenden ein Schutzgas (z.B. Helium oder Argon) im Thermometer, um den Phasenübergang zu verhindern. Helium und Argon haben sehr niedrige Siedepunkte und bleiben gasförmig bei den Kalibriertemperaturen, was die Stabilität der Messungen gewährleistet.
- Gasdichte Handgriffe: Unsere Thermometer sind mit gasdichten Handgriffen ausgestattet. Diese Handgriffe sind so konzipiert, dass sie vollständig abgedichtet sind und das Schutzgas im Inneren halten. Dadurch wird verhindert, dass Umgebungsfeuchte oder Luft in das Thermometer eindringen und den Phasenübergang auslösen.

In eigener Sache
Referenzthermometer für extrem niedrige Temperaturen
Optimal für präzise Messungen bei tiefsten Temperaturen von -200 °C bis 250 °C. Dank des Designs ohne Quarzmantel bleibt das Thermometer auch bei extremen Bedingungen stabil und bruchsicher bei Vereisung. Der kompakte Messwiderstand minimiert die Wärmeableitung und sorgt für genaue Messergebnisse. Erhältlich mit akkreditierter Kalibrierung nach DIN EN ISO/IEC 17025 (DAkkS).
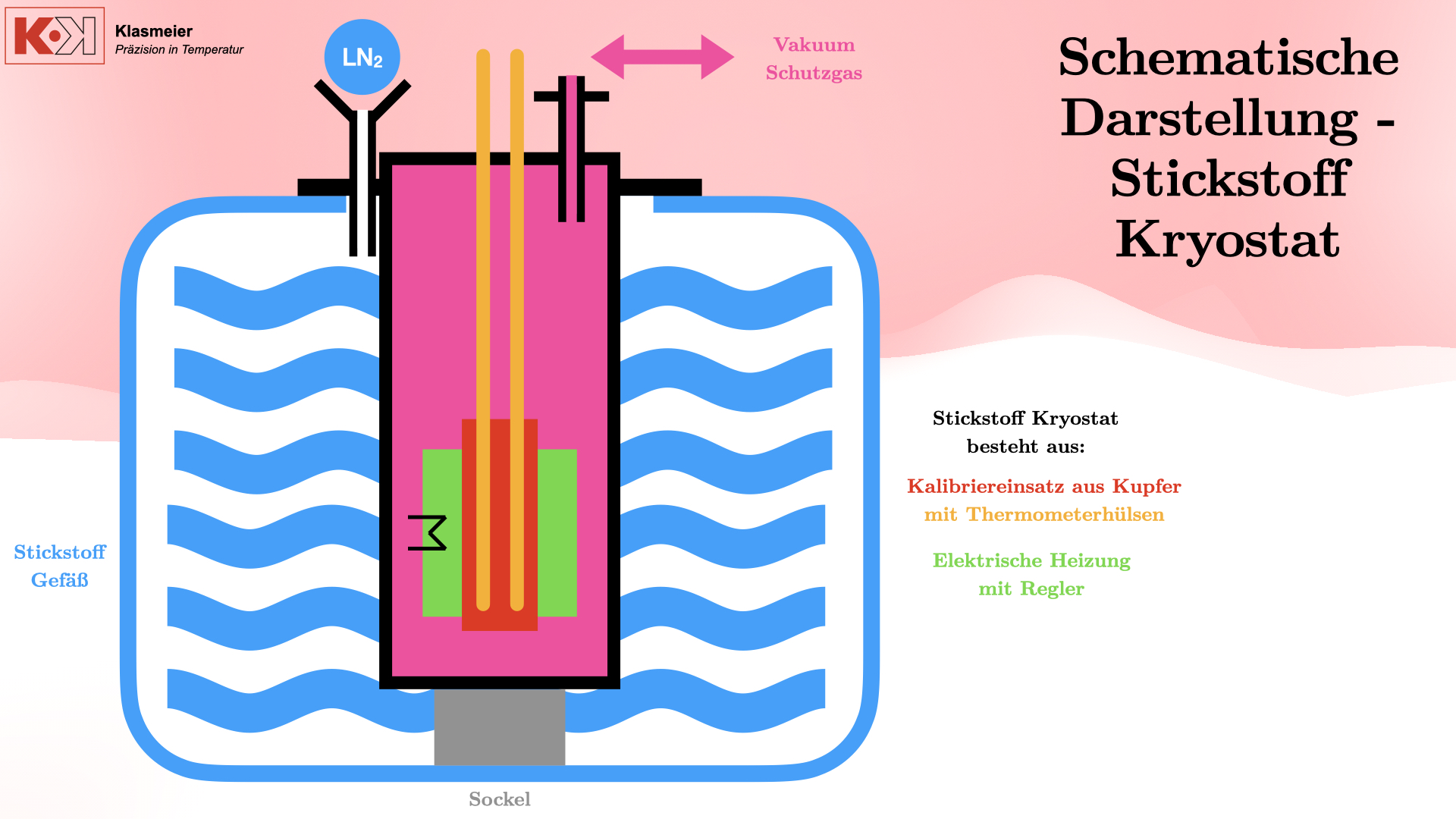
Der Stickstoff-Kryostat
Um die Lücke zwischen den klassischen Verfahren zur Kalibrierung von Thermometern und der Temperatur-Fixpunktkalibrierung zu schließen, können Stickstoff-Kryostate verwendet werden.
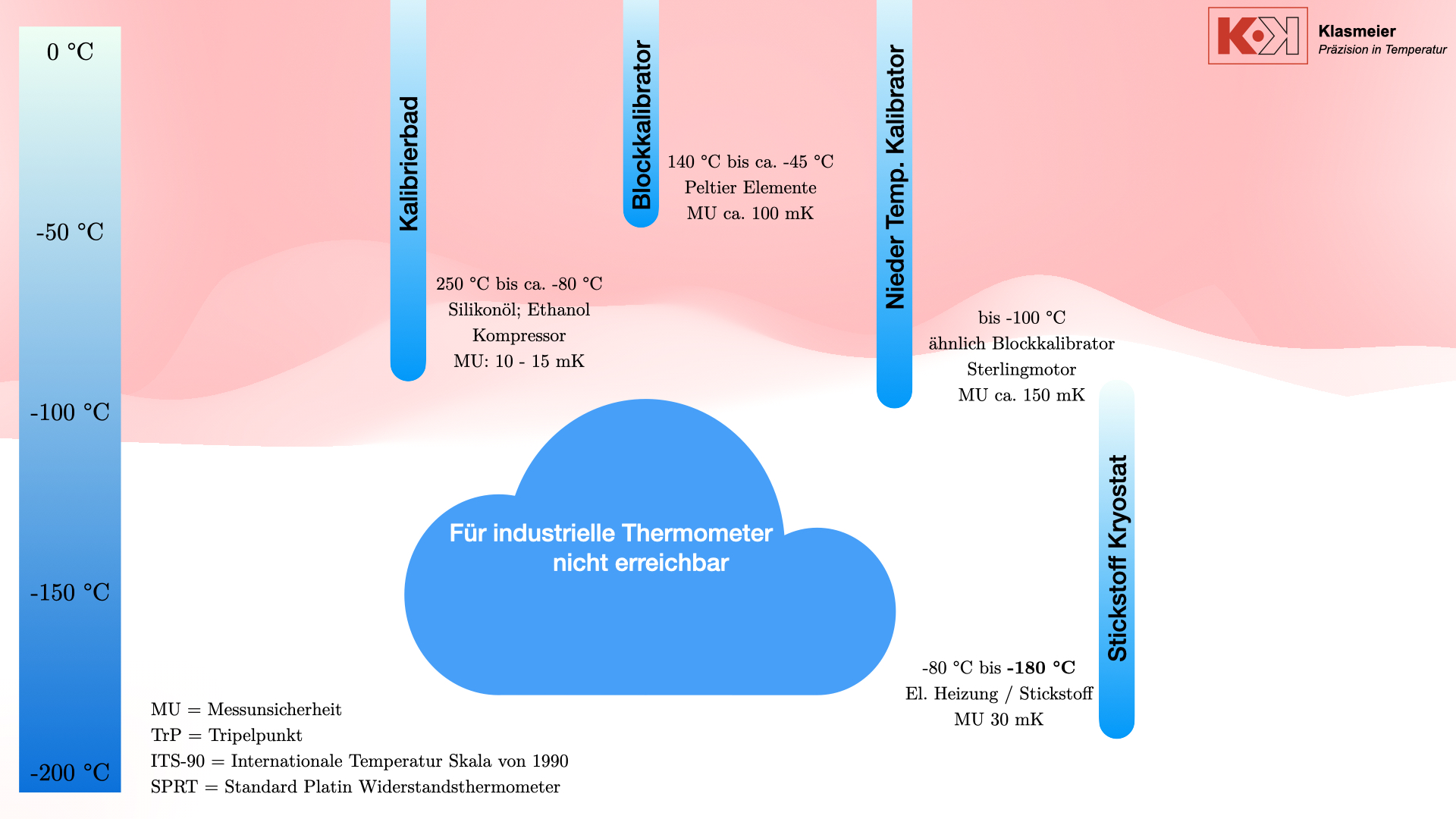
Dieses System verwendet flüssigen Stickstoff und eine elektrische Heizung, um stabile Kalibriertemperaturen zu erreichen und bietet damit eine flexible und präzise Lösung für die Kalibrierung bei extrem niedrigen Temperaturen.

Aufbau und Funktionsweise
Der Stickstoff-Kryostat besteht aus folgenden Komponenten:
- Edelstahlgefäß: Gasdicht verschweißt und auf einem Sockel platziert, damit es von flüssigem Stickstoff umspült werden kann.
- Kalibriereinsatz aus Kupfer: Enthält zwei Thermometerhülsen aus Edelstahl, die gasdicht verschweißt sind.
- Elektrische Heizung: Um den Stickstoff gezielt zu erhitzen und stabile Kalibriertemperaturen zu erreichen.
- Vakuum- oder Schutzgassystem: Um den Phasenübergang von Luft zu verhindern und eine stabile Kalibriertemperatur zu gewährleisten.
Durch das Erzeugen eines Vakuums oder das Füllen mit Schutzgas wie Argon oder Helium wird verhindert, dass der Phasenübergang der Luft die Kalibrierung beeinflusst. Der Kryostat kann Temperaturen von -80 °C bis -180 °C mit einer Messunsicherheit von 30 mK erreichen.
Evakuierung des Kryostaten:
Der Phasenübergang von Luft muß nicht nur bei den Thermometern, sondern auch bei den Kryostaten unterbunden werden. Deswegen muß der Kryostat ebenfalls mit Schutzgas gefüllt werden.
- Evakuierung: Das Edelstahlgefäß des Kryostaten wird zunächst evakuiert, um die Luft zu entfernen.
- Füllen mit Schutzgas: Anschließend wird das Gefäß mit Schutzgas (z.B. Helium) gefüllt.
- Abdichtung: Die Thermometer werden in die gasdichten Hülsen eingeführt und mit speziellen Teflondichtungen abgedichtet, um das Eindringen von Feuchtigkeit zu verhindern.
- Kalibrierung: Das Thermometer wird im Stickstoff-Kryostaten auf die gewünschte Kalibriertemperatur gebracht und kalibriert.

In eigener Sache
Stickstoff-Kryostat zur Thermometer Kalibrierung bis -196 °C
Kalibrieren Sie Ihre Thermometer und Thermoelemente präzise und kontaminationsfrei im Temperaturbereich von -80 °C bis -180 °C! Der Stickstoff-Kryostat der Firma ISOTECH ermöglicht Ihnen die freie Wahl der Kalibrierpunkte, perfekt angepasst an Ihre Anforderungen. Dank des fest verbauten Kalibriereinsatzes, der Platz für drei Thermometer bietet, erreichen Sie höchste Genauigkeit bei der Kalibrierung – ohne Kontakt mit dem Stickstoff.
Praktische Anwendung und Integration
Stickstoff-Kryostate können in bestehende Kalibriereinrichtungen integriert werden. So kann in Kombination von Kryostaten und Kalibrierbädern ein Thermometer, was nicht die Anforderungen an eine Kalibrierung an Temperatur-Fixpunkten erfüllt, komplett im negativen Temperaturbereich kalibriert werden.
Der Stickstoffverbrauch für eine vollständige Kalibrierung beträgt etwa 60 Liter, was für eine Woche Betrieb ausreicht. Die initiale Abkühlzeit des Kryostaten liegt bei etwa sechs Stunden, und Temperaturänderungen dauern je nach Temperatur-Differenz bis zu vier Stunden.
Beispiel einer Kalibrierung
Ein typischer Kalibrierungsprozess umfasst mehrere Schritte:
- Initiale Abkühlung: Der Kryostat wird mit flüssigem Stickstoff befüllt und auf -196 °C abgekühlt.
- Temperaturstabilisierung: Die gewünschte Kalibriertemperatur wird durch gezieltes Heizen gegen den Siedepunkt von Stickstoff erreicht.
- Kalibrierung: Das zu kalibrierende Thermometer wird in die Thermometerhülsen eingeführt und die Temperatur wird gemessen und verglichen. Temperaturdifferenzen oder Wertepaare werden festgestellt und das zu prüfende Thermometer kalibriert.

