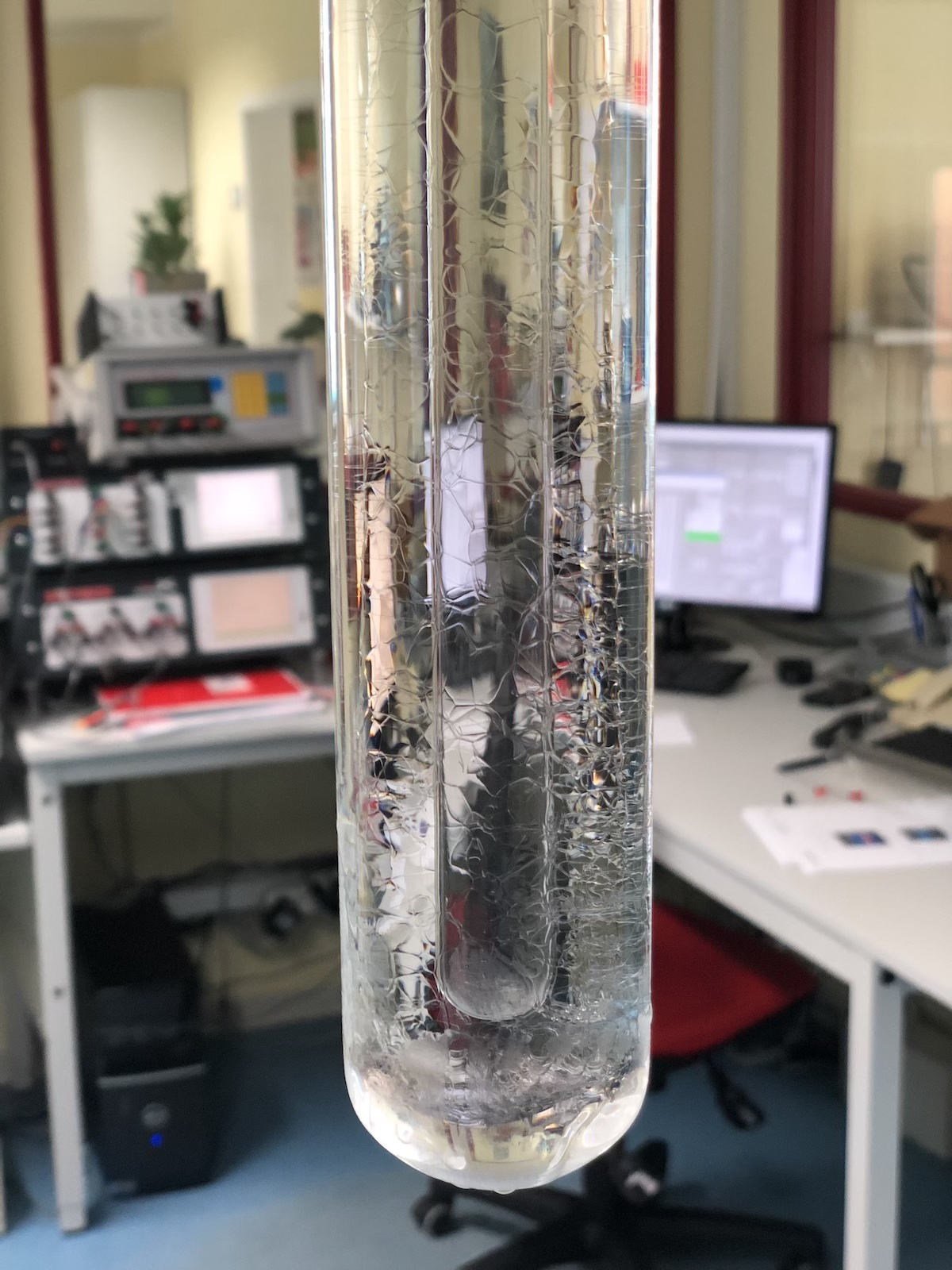
The water triple point is a specific state where water exists simultaneously in all three phases (solid, liquid, and gaseous) in thermodynamic equilibrium. The water triple point is an important reference temperature for calibrating temperature sensors and thermometers.
Table of Contents
Basic knowledge
The water triple point occurs at a specific pressure, typically defined as 611.657 Pa (0.0060366 atm). At this pressure, the temperature at which the three phases of water are in equilibrium is exactly 273.16 Kelvin (0.01°C). This value is the basis for the Kelvin temperature scale, which is an absolute scale where zero is the absolute zero point, corresponding to the theoretical temperature at which all molecules and atoms of a substance have their minimum energy.
The definition of the water triple point provides a precise and reproducible reference temperature used in the calibration of thermometers and temperature sensors. It is also an important reference temperature for other scientific applications, such as determining material constants and developing thermodynamic models.
The water triple point in the phase diagram
The water triple point can be identified on the phase diagram of water. This diagram represents the various states (phases) of water under different pressure and temperature conditions. Water can exist in three main states: solid (ice), liquid (water), and gaseous (vapor). The phase diagram of water shows under which pressure and temperature conditions each of these phases is stable.
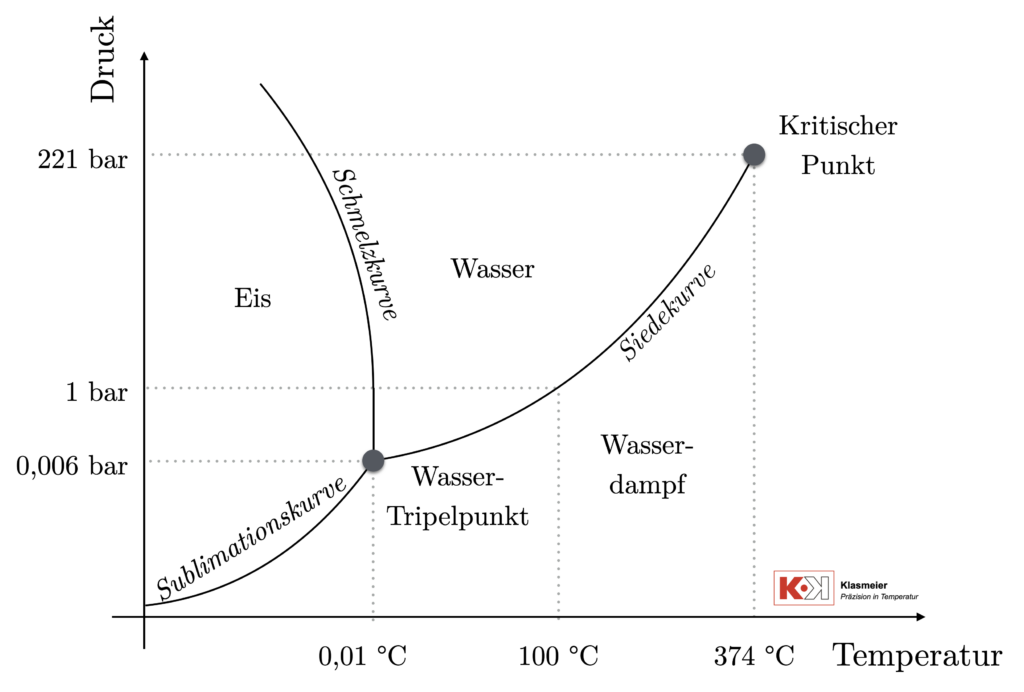
Important points and curves in the phase diagram of water:
- The water triple point This is where the curves for the solid, liquid, and gaseous states intersect. At this triple point of water, all three phases can exist simultaneously in equilibrium. For water, the triple point occurs at 0.01°C and 611.657 Pascal. Since the water triple point is defined by temperature and pressure, it is excellently suited as a temperature fixed point for calibrating thermometers.
- Melting curve: The melting curve separates the solid state from the liquid state. Along this line, ice melts into water or water freezes into ice. This phase transition can be used as a secondary fixed point for calibrating thermometers. However, it is pressure-dependent and occurs at approximately 0°C.
- Boiling curve: This line separates the liquid state from the gaseous state. Along this line, water evaporates into steam or steam condenses into water. The boiling point can also be used as a secondary fixed point for calibrating thermometers. The boiling point of water at atmospheric pressure is approximately 100°C.
- Sublimation curve: This line separates the solid state from the gaseous state. Along this line, water can sublimate directly from solid (ice) to gaseous (vapor) or vice versa, without passing through the liquid state.
- Critical point: This point marks the end of the boiling curve. Beyond this point, the liquid and gaseous phases can no longer be distinguished, and they become a supercritical fluid. For water, this point is at about 374°C and a pressure of 22.06 MPa.
Particularly noteworthy in the phase diagram of water is the negative slope of the melting curve. This means that under increasing pressure, the melting point of ice decreases. This is unusual and differs from most other substances. It also explains why ice floats on water.
In eigener Sache
Precise Temperature Fixed Points for Thermometers
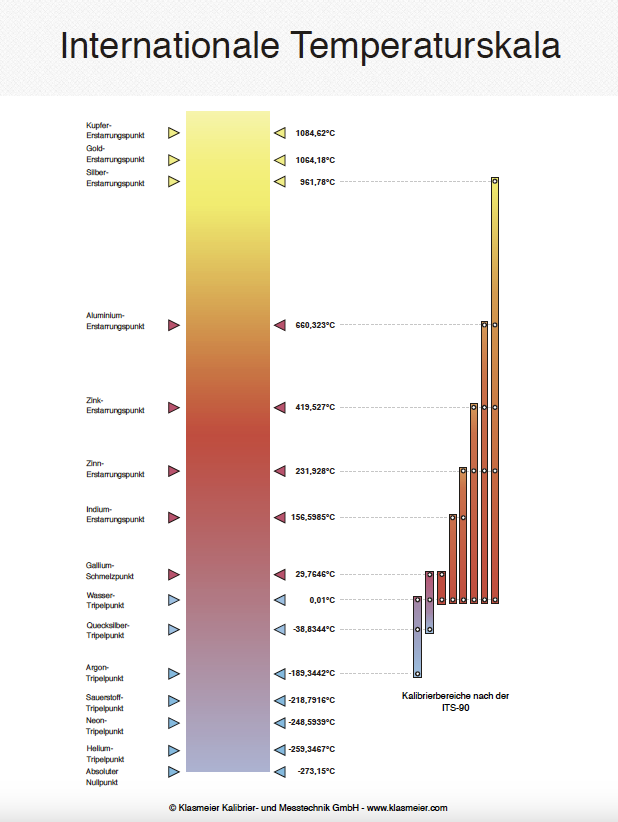
The company Klasmeier offers high-precision temperature fixed points according to ITS-90 for the calibration of thermometers from the company ISOTECH. These fixed point cells are available in various designs and enable reliable and reproducible measurement results in laboratories and research applications.
Commissioning a Water Triple Point Cell – “Thermometer Well Method”
The standard method for preparing an ice mantle around the thermometer well of a water triple point cell is the “Thermometer Well Method”. Here, the ice mantle is formed from the inside out by cooling the thermometer well. Depending on the coolant used (crushed solid CO2, immersion cooler with heat pipe, liquid nitrogen-cooled rod, or liquid nitrogen), various variants can be applied, which can be summarized as follows:
- Crushed solid CO2: The thermometer well is filled with crushed solid CO2 up to the water surface in the cell, and such CO2 is added until a mantle of the desired thickness is formed. About 1 ml of ethanol is added before the CO2 to promote heat transfer and a thicker mantle at the bottom.
- Immersion cooler with heat pipe: Initially, about 1 ml of ethanol and 5 ml of finely crushed solid CO2 are added to the thermometer well to promote crystal nucleation and a thicker mantle at the bottom, and to protect the water in the cell from supercooling. The immersion cooler is then inserted into the thermometer well and the space between the thermometer well and heat pipe is filled with ethanol. A heat conduction cycle begins, and the ice mantle forms.
- Liquid nitrogen-cooled rod: The thermometer well is filled with ethanol and a metal rod pre-cooled in liquid nitrogen is inserted. Several repetitions are necessary to produce a sufficient mantle.
- Liquid nitrogen: This variant can have different sub-variants. Usually, the cold is conducted into the thermometer well of the water triple point cell through a heat pipe with a cooler containing the liquid nitrogen.
In all the variants described above, the water triple point cell must be pre-cooled to a temperature close to 0 °C. During the cooling process, care must be taken to ensure that no solid ice bridge forms on the upper surface. It is also essential to remove all water from the thermometer well before preparing the ice mantle, for example by rinsing with high-purity ethanol.

In eigener Sache
Water Triple Point Maintenance Bath
The Water Triple Point Maintenance Bath from ISOTECH enables the precise commissioning and maintenance of up to four water triple point cells at a temperature of 0.01 °C. It offers vibration-free, fully automatic operation and is energy-efficient. Suitable for laboratories and calibration tasks.
The time required for the formation of an ice mantle depends on the chosen variant: about 30 minutes for variants 1 and 3, 60 minutes or more for variant 2, 10 to 120 minutes for variant 4.
With an alternative, non-standardized method known as the “Mush Method”, the ice mantle is formed from the outside in. Although this method has practical advantages (It can be carried out in an industrial temperature block calibrator.) and has been shown to agree with the “Thermometer Well Method” to within 0.1 mK, its use is normally limited to checking the stability of reference SPRTs in secondary temperature calibration laboratories.
Commissioning a Water Triple Point Cell – “Mush Method”
As an alternative to the “Thermometer Well Method”, a water triple point cell can also be commissioned more economically efficiently in a temperature block calibrator. This method is also called the “Mush Method” and also allows for extremely precise temperature measurement and can be used in many scientific and industrial applications.
The first step is to carefully place the water triple point cell into the calibration volume of the temperature block calibrator. Then, turn on the temperature block calibrator and set the target value to -8°C. It is important to monitor the temperature with a thermometer in the measurement channel of the water triple point cell during the cooling process. In this context, we recommend using a liquid in the measurement channel of the water triple point cell to optimize heat transfer, for example, a mixture of water and ethanol.

In eigener Sache
Temperature block calibrator for commissioning water triple point cells
With the VENUS temperature block calibrator from ISOTECH, you can commission water triple point cells and calibrate temperature sensors with them.
When the temperature in the water triple point cell reaches -6°C, the process of ice mantle formation can be initiated. For this purpose, remove the water triple point cell from the temperature block calibrator, gently shake it, and observe how the ice forms from the water surface to the bottom of the cell. This process leads to an increase in the temperature in the cell to the triple point of water, which is at 0.01°C.
To ensure the stability of the ice-water mixture, the cell can be cooled for an additional 45 minutes at -6°C. It is important to note that during measurements at the water triple point, care must be taken to ensure that the ice mantle does not freeze to the measurement channel or the wall of the cell. If this happens, you can thaw the ice mantle in the cell with a metal rod and warm the outer mantle using hand heat.
Finally, to maintain the ice mantle of the water triple point cell, the set point of the temperature block calibrator should be set to -1°C. This ensures that the ice mantle in the cell remains stable and allows accurate measurements to be made.
In summary, commissioning a water triple point cell in a temperature block calibrator is a complex but feasible process. By carefully following the steps presented here, you can perform accurate and reliable temperature measurements and calibrate thermometers.
How water triple points save costs and minimize risks
In the world of precision thermometry, where accurate temperature measurements are essential for a variety of applications, water triple point cells and gallium melting point cells play a key role.
The use of these temperature fixed points in temperature laboratories, especially for users of Standard Platinum Resistance Thermometers (SPRT) or high-quality industrial Platinum Resistance Thermometers (PRT), can save costs and minimize risks.
The value of regular checks
While the use of these precision thermometers relies on external calibration by specialized temperature calibration laboratories, the question arises: What happens between calibration cycles? Thermometers can be impaired during transport or improper handling, leading to changes in their values (thermometer drift). Such a change, only detected at the next calibration, can have serious consequences, such as the potential invalidity of all previous measurements, which would require a recall of all calibrated devices. Such incidents can severely impair confidence in temperature calibration and cause significant costs. However, these risks can be minimized through regular checks of the thermometers at the water triple point and the gallium melting point.
Checks at the water triple point
The calibration certificate of an accredited temperature calibration laboratory will state the last value of the water triple point. After receiving a calibrated thermometer, it should be checked at the water triple point and the result compared with this value. Such a check allows for a measurement uncertainty of less than 0.001°C and is an essential step in ensuring the reliability of the thermometer between calibrations.
The gallium melting point: Another way to assess the reliability of thermometers
The gallium melting point allows the measurement of the resistance value of a thermometer at 29.7646°C. It is easy to handle and offers very low measurement uncertainties. The combination of the gallium melting point and water triple point allows the calculation of the resistance ratio (WGA), a crucial value for assessing the reliability of the thermometer.
This so-called W-value is calculated from the current resistance – in our case, the resistance at the gallium melting point R(GA) – and the last known resistance value of the thermometer at the water triple point R(WTP):
W(GA) = R(GA) / R(WTP)
The Benefit of W-Value Calculation
The W-value is an important parameter in temperature measurement as it essentially calculates the slope of a thermometer’s characteristic curve. The purer the platinum of the temperature sensor in the resistance thermometer, the higher this W-value.
There are certain scenarios that can lead to changes in the thermometer’s performance. Let’s assume the resistance value of the thermometer at the water triple point increases, but the W-value at the gallium melting point (WGA) remains constant. In such cases, this indicates that the characteristic curve has shifted in parallel. This is the classic thermometer drift. This drift effect can occur due to mechanical and thermal stresses. The good news is that such changes are often reversible, and calibration can correct these effects and is therefore meaningful.

In eigener Sache
Calibration of Temperature Fixed Points
The company Klasmeier offers accredited calibrations according to DIN EN ISO/IEC 17025 (DAkkS) for temperature fixed points. These are performed by comparison with high-precision reference cells and standard platinum resistance thermometers (SPRT). Various temperature fixed points according to ITS-90, such as water triple point, mercury and gallium melting points, are calibrated.
However, if the W-value changes (typically decreases), it is an indication that the thermometer is contaminated. Unfortunately, such changes are often not reversible, and in many cases, the thermometer can no longer be calibrated.
Regular checks of the WGA can determine how the thermometer is stressed in daily operation. These checks can therefore provide valuable decision-making assistance as to whether calibration is meaningful or not. They can also help to maximize the lifespan and accuracy of the thermometer and avoid unexpected failures.
Advantages of Regular Measurement at the Water Triple Point and W-Value
Regular measurement of the values R(WTP) (resistance at the water triple point) and W(GA) (ratio of resistance at the water triple point to resistance at the gallium melting point) offers a number of advantages:
Cost savings: Through regular self-checks, users can extend the calibration periods in accredited, external laboratories, leading to significant cost savings.
Increased confidence: Regular checks and thus confirmation of the accuracy and reliability of the thermometers strengthens the confidence level in the laboratory.
Error prevention: By identifying and correcting potential problems, erroneous measurements can be avoided, which could otherwise lead to serious consequences.
Furthermore, the use of gallium melting points and water triple points in temperature laboratories allows for significant risk minimization by reducing the transport of thermometers for external calibrations. This not only saves transport costs but also minimizes the risk of damage to the thermometers.
It is advantageous for users of SPRTs and PRTs to have access to their own water triple point and gallium melting point cells. They allow for regular checks and adjustments, increase confidence in the reliability of the thermometers, and can reduce the need for external calibrations and associated costs and risks. In a world where precision is crucial, water triple points and gallium melting points offer an effective way to ensure accuracy and reliability in thermometry.
Quellen
- Walter Blanke: Die Internationale Temperaturskala von 1990: ITS-90
- Hering, Martin, Stohrer: Physics for Engineers
- Beiz, Grote: Dubbel – Handbook for Mechanical Engineering
- Thomas Klasmeier: Table Book “Temperature”, Edition 3
- Guide to the Realization of the ITS-90 – Triple Point of Water – Bureau International des Poids et Mesures