Accurate temperature measurements are crucial in many industries, especially in pharmaceuticals, cryogenics, and scientific research.
Calibrating thermometers at extremely low temperatures, ranging from -80°C to -180°C, poses a significant technical challenge. This article describes the technical possibilities for calibrating thermometers at these extremely low temperatures.
Table of Contents
Calibration Methods at Low Temperatures
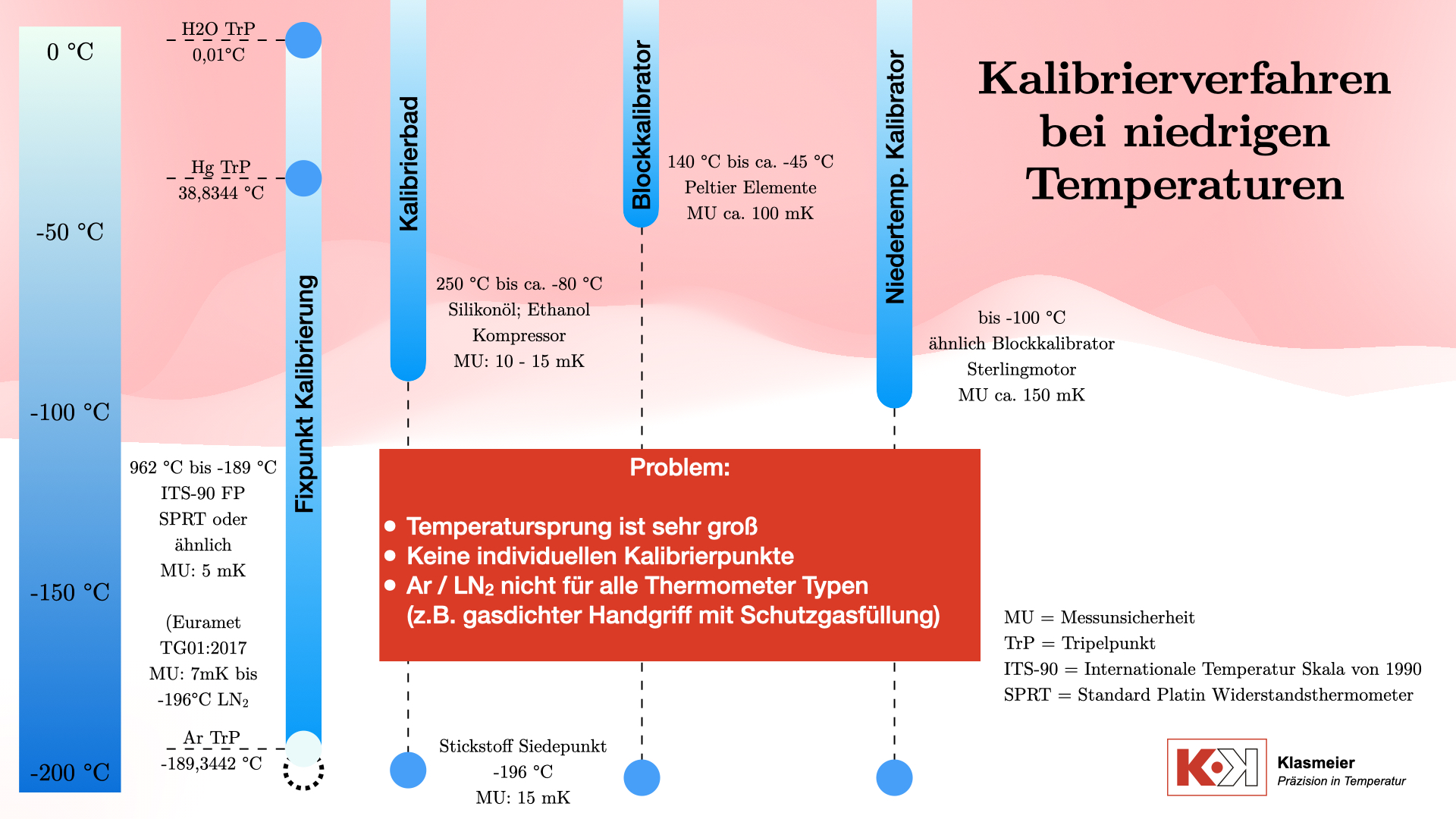
Calibration Baths
The classical method for calibrating thermometers at low temperatures uses calibration baths. These typically consist of two working chambers:
- Calibration Medium Chamber: This chamber tempers and circulates the calibration medium.
- Calibration Chamber: This chamber is where the thermometer is calibrated.
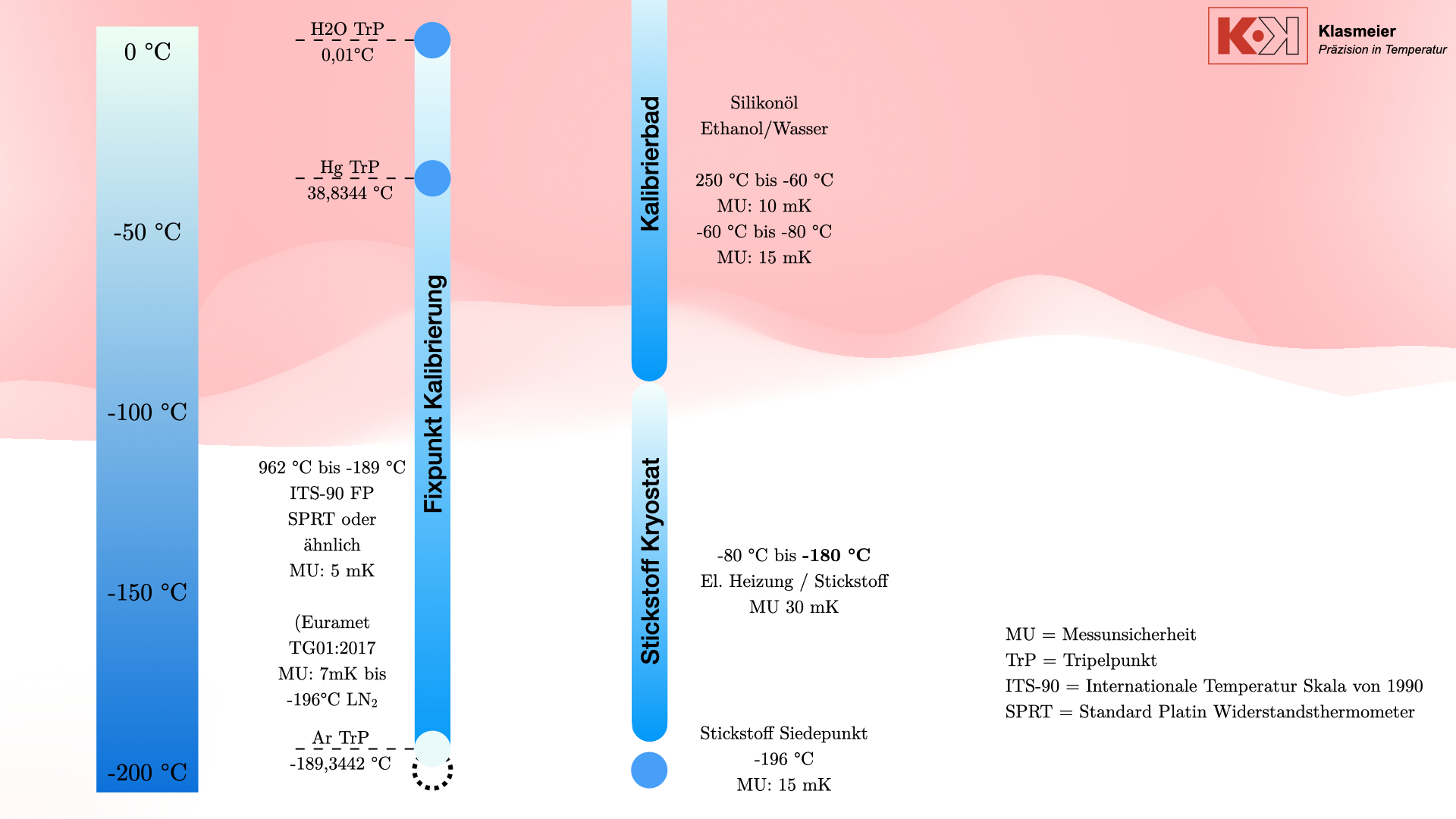
The most common calibration medium is silicone oil, which is used for temperatures up to 250°C. For lower temperatures, down to about -80°C, ethanol is used. Some calibration baths can reach even lower temperatures, but the effort often does not justify the benefit. Calibration baths are cooled using a compressor, which always operates at 100% capacity, with an electric heater tempering against this full cooling power. Thus, the calibration temperature is achieved not by cooling alone but by heating against the cooling. This method offers significantly higher control accuracy and stability. However, the disadvantage is the considerable effort required to maintain a stable temperature.
The achievable measurement uncertainty for these calibration baths is typically between 10 mK and 15 mK.
Temperature Dry-Block Calibrators
Temperature dry-block calibrators are widespread in the industry due to their user-friendliness. They operate with Peltier elements and can reach temperatures up to about -70°C below ambient temperature. This limits the temperature range in the negative zone to about -45°C when used in laboratory environments. Their measurement uncertainty is around 50 mK to 100 mK, which is sufficient for many industrial applications. However, this measurement uncertainty is not accurate enough for use in calibration laboratories.
Low-Temperature Calibrators
Low-temperature calibrators are similar in design to standard temperature dry-block calibrators but do not use Peltier elements. Instead, they use Stirling engines to cool the calibration volume, allowing them to reach temperatures as low as -100°C. These devices have the advantage of not requiring liquids as a calibration medium. However, they are relatively expensive and have a high measurement uncertainty of about 150 mK.
Calibration at the Secondary Temperature Fixed Point of Nitrogen
In addition to classical calibration methods, there is the possibility of calibrating thermometers at the secondary temperature fixed point of nitrogen. The boiling point of nitrogen is -196°C, a well-defined secondary fixed point that can technically be used for calibration. However, this method has some drawbacks.
Although calibration at the nitrogen boiling point is technically feasible and very cost-effective, this point is not defined in the International Temperature Scale of 1990 (ITS-90). This means that the calibration does not achieve the same accuracy and international recognition as temperature fixed-point calibration according to ITS-90.
Another problem is the limited number of calibration points. Using the nitrogen boiling point provides too few calibration points. The temperature gap from about -80°C or -100°C in, for example, a calibration bath to the nitrogen boiling point of -196°C is too large to calculate a precise thermometer calibration curve. Thus, accurate and continuous calibration over the entire temperature range is not possible.
Therefore, calibration at the nitrogen boiling point is not recommended, despite being technically feasible.
Temperature Fixed-Point Calibration According to ITS-90
The most precise method for calibrating thermometers is the fixed-point calibration according to the International Temperature Scale of 1990 (ITS-90). This method includes the following fixed points in the negative temperature range:
- Water Triple Point: 0.01 °C
- Mercury Triple Point: -38.8344 °C
- Argon Triple Point: -189.3442 °C
Additionally, the nitrogen boiling point at -196°C can be used as a secondary temperature fixed point. According to the EURAMET TG 01:2017 guideline, the ITS-90 thermometer curve (deviation function) can be extrapolated down to -196°C.
A major issue in calibrating at extremely low temperatures is the handling of the thermometers. Special gas-tight handles and protective gas fillings are necessary to ensure precise measurements.
While this method covers the entire temperature range, not all thermometers are suitable for fixed-point calibration. This leads to a gap in calibration capability for certain temperature ranges and types of thermometers. Thermometers must be designed to be calibratable at temperature fixed points, which means they must have a minimum length and a suitable diameter at the protective tube.
Another significant limitation is that thermometers must be exposed to the extremely low temperature of around -189°C at the argon triple point. For this to be possible, certain design requirements must be met. For example, the thermometer’s measurement channel must be free of air because the phase transition of oxygen would make calibration impossible. This fact restricts many thermometers. A thermometer that can only be used down to -150°C, for example, cannot be calibrated at negative temperature fixed points.

Advertisement
Precise calibration at extremely low temperatures
With Klasmeier’s calibration services, you can have your thermometers calibrated within the temperature range of -180 °C to -80 °C, as well as at fixed points of -189 °C and -196 °C, according to DIN EN ISO/IEC 17025 (DAkkS). Klasmeier provides precise and reliable measurement results that meet your specific requirements.
Challenge: The Phase Transition of Air
A central problem when calibrating at extremely low temperatures is the phase transition of air. At temperatures below -180°C, the components of air (mainly nitrogen and oxygen) begin to condense and eventually liquefy. This leads to significant measurement problems as the gas mixture exists in different phases.
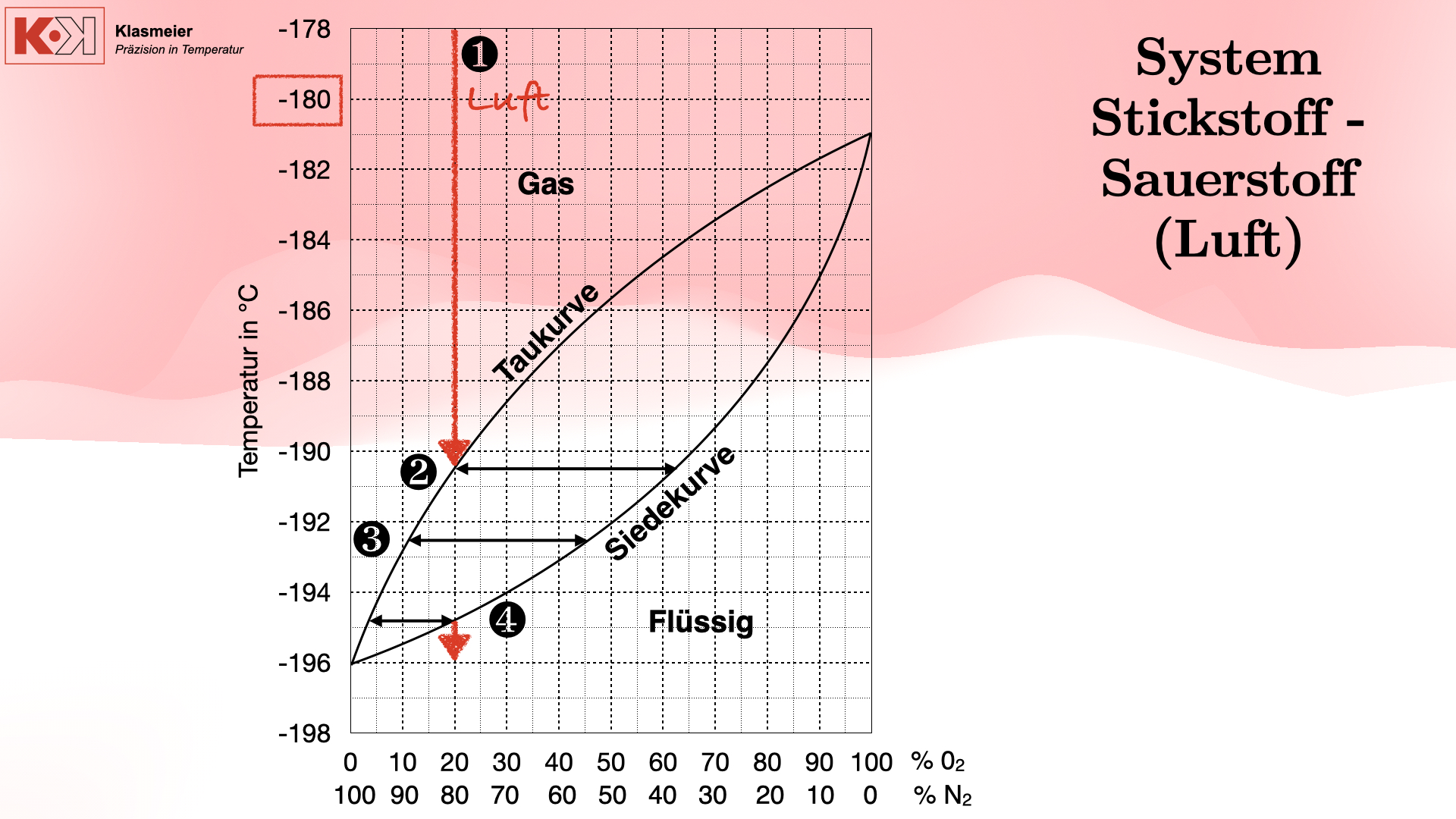
Phase Transition of Air:
- Dew Point: Upon cooling, droplets of liquid oxygen and nitrogen form, with the dew point not being uniform due to the gas mixture.
- Boiling Point: Further cooling leads to complete liquefaction of the air components, though with different compositions.
- Condensation: Finally, the air fully condenses into a liquid mixture of 80% nitrogen and 20% oxygen.
This phase transition can cause thermometers passing through this range to provide unreliable measurements due to the varying phase transitions they undergo.
Solution: Protective Gas and Gas-Tight Handles
To prevent the phase transition of air within the thermometer, a protective gas filling can be used:
- Protective Gas Filling: We use a protective gas (e.g., helium or argon) within the thermometer to prevent the phase transition. Helium and argon have very low boiling points and remain gaseous at calibration temperatures, ensuring measurement stability.
- Gas-Tight Handles: Our thermometers are equipped with gas-tight handles. These handles are designed to be fully sealed, keeping the protective gas inside and preventing ambient moisture or air from entering and triggering a phase transition.

Advertisement
Reference thermometer for extremely low temperatures
Ideal for precise measurements at ultra-low temperatures from -200 °C to 250 °C. The quartz-free design ensures stability even in extreme conditions, preventing breakage during freezing. Its compact temperature detector minimizes heat dissipation, delivering highly accurate results. Available with accredited calibration according to DIN EN ISO/IEC 17025 (DAkkS).
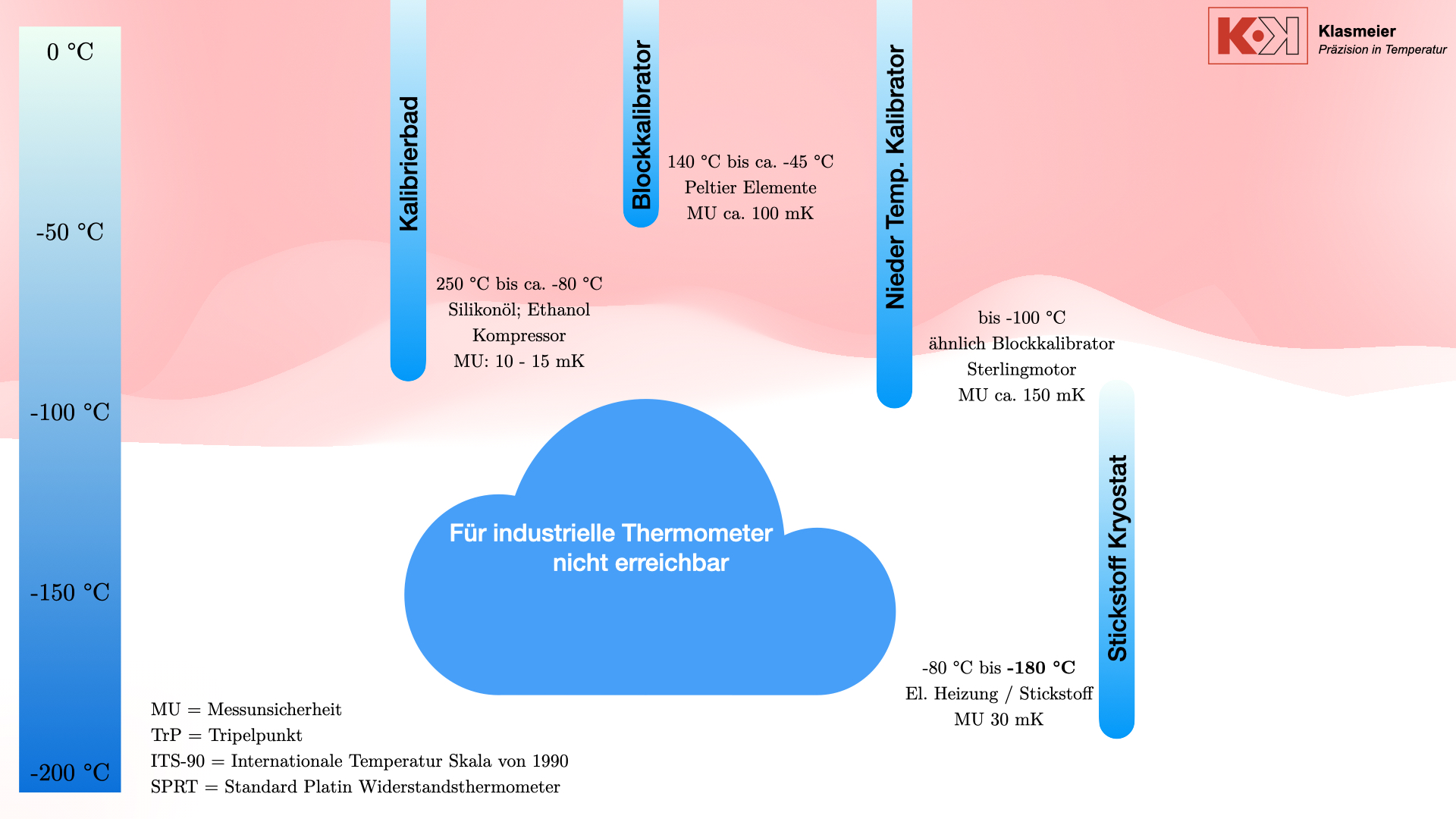
The Nitrogen Cryostat
To bridge the gap between classical calibration methods and fixed-point calibration, nitrogen cryostats can be used.
This system uses liquid nitrogen and an electric heater to achieve stable calibration temperatures, providing a flexible and precise solution for calibration at extremely low temperatures.

Components and Function
The nitrogen cryostat is composed of the following components:
- Stainless Steel Vessel: Gas-tight welded and placed on a base to be surrounded by liquid nitrogen.
- Copper Calibration Insert: Contains two stainless steel thermometer sleeves, gas-tight welded.
- Electric Heater: Heats the nitrogen to achieve stable calibration temperatures.
- Vacuum or Protective Gas System: Prevents the phase transition of air and ensures a stable calibration temperature.
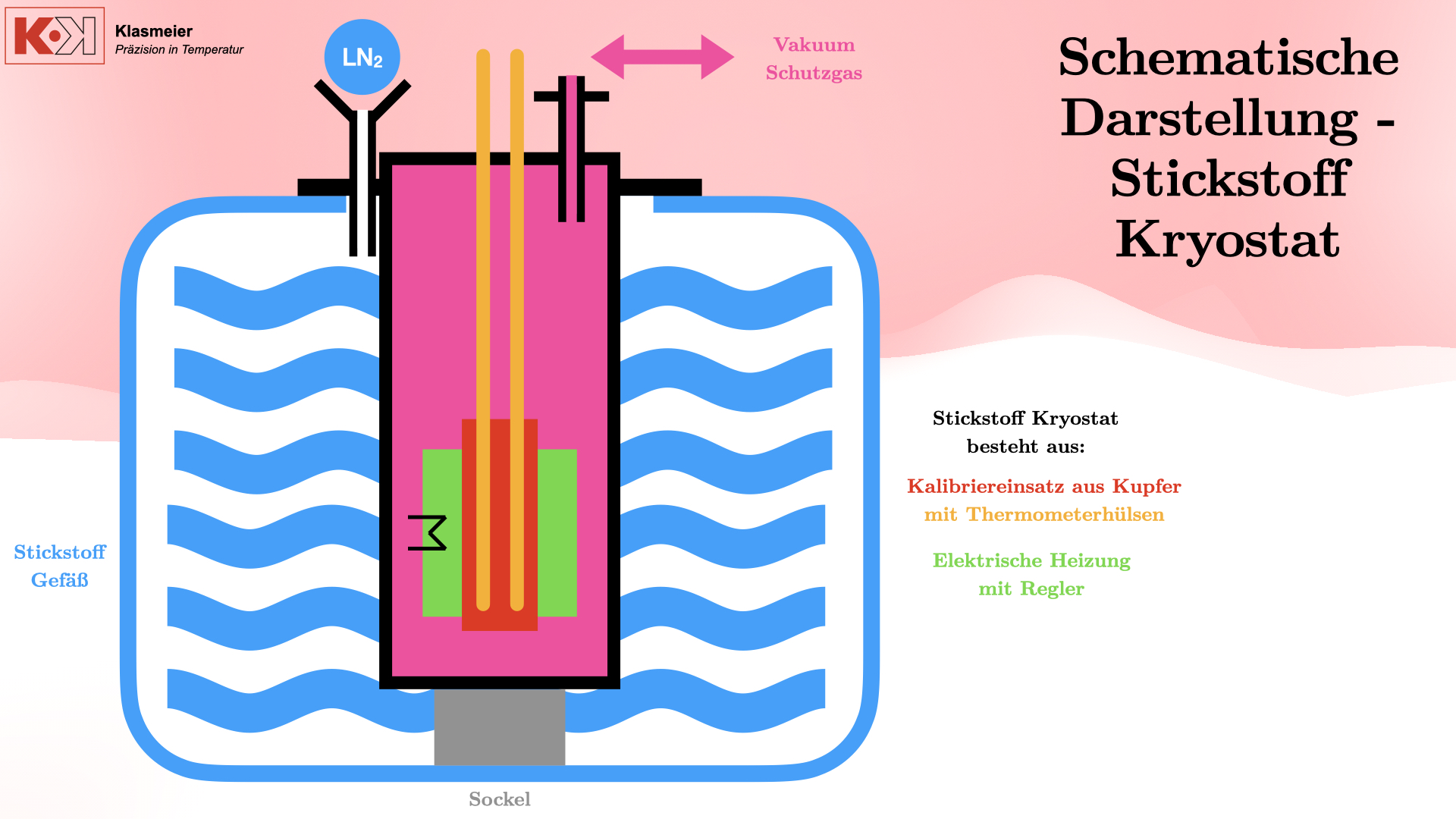
By creating a vacuum or filling with protective gas such as argon or helium, the phase transition of air is prevented, ensuring that the calibration is not affected. The cryostat can reach temperatures from -80°C to -180°C with a measurement uncertainty of 30 mK.
Evacuation of the Cryostat:
The phase transition of air must be prevented not only in thermometers but also in cryostats. Therefore, the cryostat must also be filled with protective gas.
- Evacuation: The stainless steel vessel of the cryostat is initially evacuated to remove air.
- Filling with Protective Gas: The vessel is then filled with protective gas (e.g., helium).
- Sealing: The thermometers are inserted into the gas-tight sleeves and sealed with special Teflon seals to prevent moisture ingress.
- Calibration: The thermometer is brought to the desired calibration temperature in the nitrogen cryostat and calibrated.
Practical Application and Integration
Nitrogen cryostats can be integrated into existing calibration facilities. By combining cryostats and calibration baths, a thermometer that does not meet the requirements for fixed-point calibration can be fully calibrated in the negative temperature range.
The nitrogen consumption for a complete calibration is about 60 liters, sufficient for a week’s operation. The initial cooling time of the cryostat is about six hours, and temperature changes take up to four hours, depending on the temperature difference.
Example of a Calibration Process
A typical calibration process involves several steps:
- Initial Cooling: The cryostat is filled with liquid nitrogen and cooled to -196°C.
- Temperature Stabilization: The desired calibration temperature is achieved by targeted heating against the boiling point of nitrogen.
- Calibration: The thermometer to be calibrated is inserted into the thermometer sleeves, and the temperature is measured and compared. Temperature differences or value pairs are determined, and the thermometer under test is calibrated.



Comments
One response to “Calibration of Thermometers at Extremely Low Temperatures (-80°C to -180°C)”
[…] 🇩🇪 | 🇬🇧 […]